Arenes or Aromatic Hydrocarbons
Arenes or Aromatic Hydrocarbons: Overview
This topic covers concepts, such as, Aromatic Compounds, Benzenoid Aromatic Compounds, Aromaticity & Huckel's Rule for Aromaticity etc.
Important Questions on Arenes or Aromatic Hydrocarbons
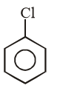
The structure of the compound 1-chloro-4-ethylcyclohexane is
In which of the following conversions oxidation is taking place
(i) propanone to propene
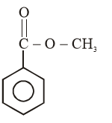
(ii) benzoic acid to benzaldehyde
(iii) bromobenzene to 1-phenylethanol
Given below are two statements :
Statement I : Tropolone is an aromatic compound and has electrons.
Statement II : electrons of group in tropolone is involved in aromaticity. In the light of the above statements choose the correct answer from the options given below:
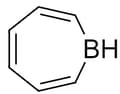
The given compound is planar, select the correct statement
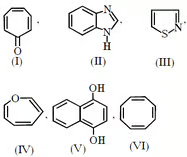
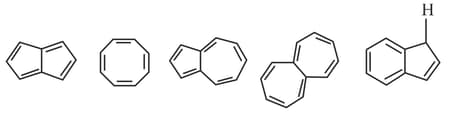
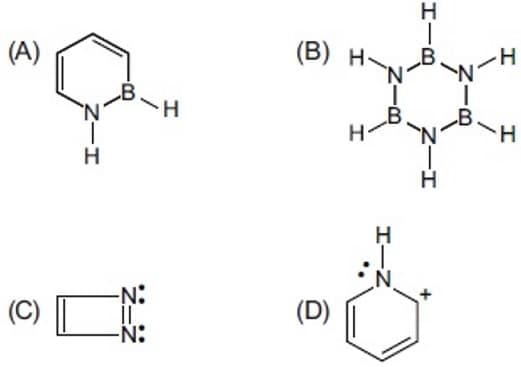
How many out of the given structure(s) are aromatic ?
The stability of benzene ring is due to the
Which one of the following is a benzenoid aromatic compound?
Among the following, the most acidic compound is :
The cyclopentadienyl cation is
Which of the following statements is not correct about the aromatic compounds?
Azulene ( 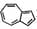 ) has dipole moment } because:
) has dipole moment } because:
Which compound have maximum dipole moment?
Determine the correct option for the following structures.
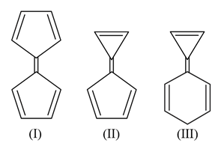
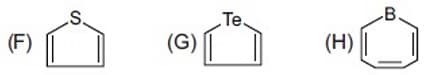
From the following molecules by considering Aromatic Behaviour, find the total number of molecules which are aromatic.
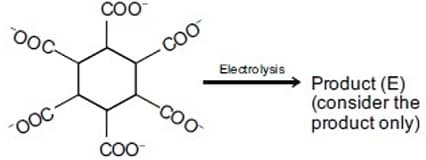
What will be the total number of sigma and pi bonds in the following structure (containing 700 benzene rings)?
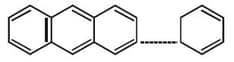
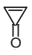
The compound has its observed dipole greater than the theoretical. Choose the most appropriate reason from the statements given.
How many isomeric phenols are possible for the molecular formula
Arrange in descending order for the rate of electrophilic substitution reaction.
(a)
(b)
(c)
(d)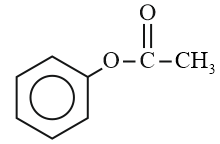
Find out the aromatic compounds from given below-
Among the following, which is not an aromatic carbocation ?